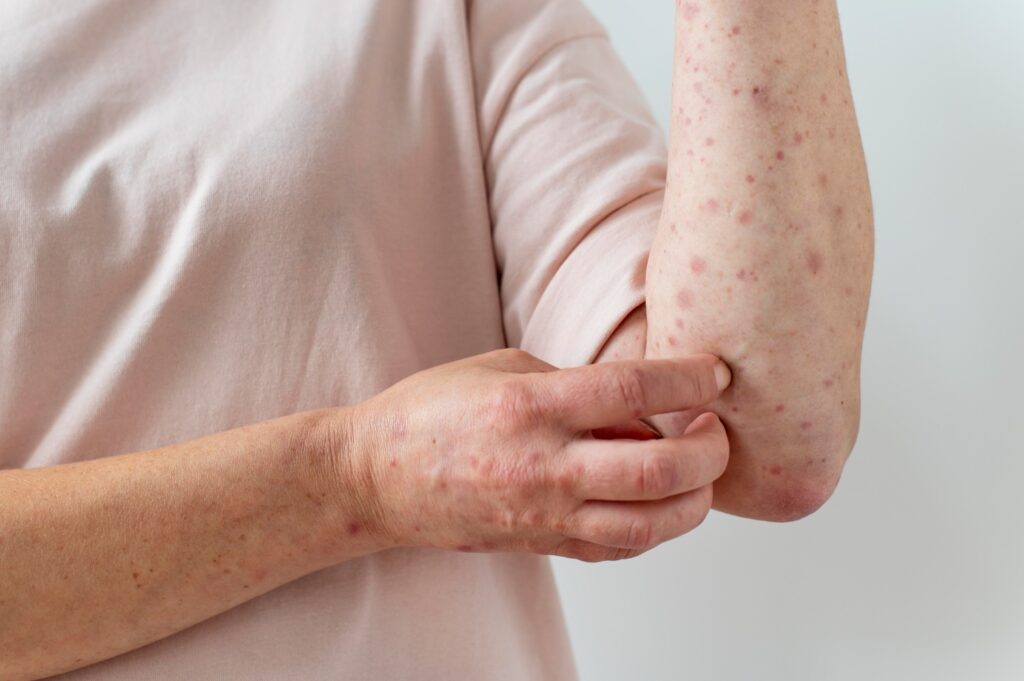
Adults aged 18 or over with a clinical diagnosis of prurigo nodularis (PN) for at least 3 months may qualify for this study. Eligible individuals must meet all inclusion criteria including having 6 or more pruriginous lesions on more than 2 different body areas. The total estimated impacted body surface area (BSA) must be 20% or less.
Participants will receive investigational topical treatment (active study drug) or placebo. Study treatment and study-related assessments will be provided at no cost. Reimbursement for study-related expenses may be provided.
Study participation will last approximately 56 weeks and involve about 12 visits to the study centre.
If interested, please contact our Research Coordinator at 416-633-0001 x 4 or e-mail [email protected].
